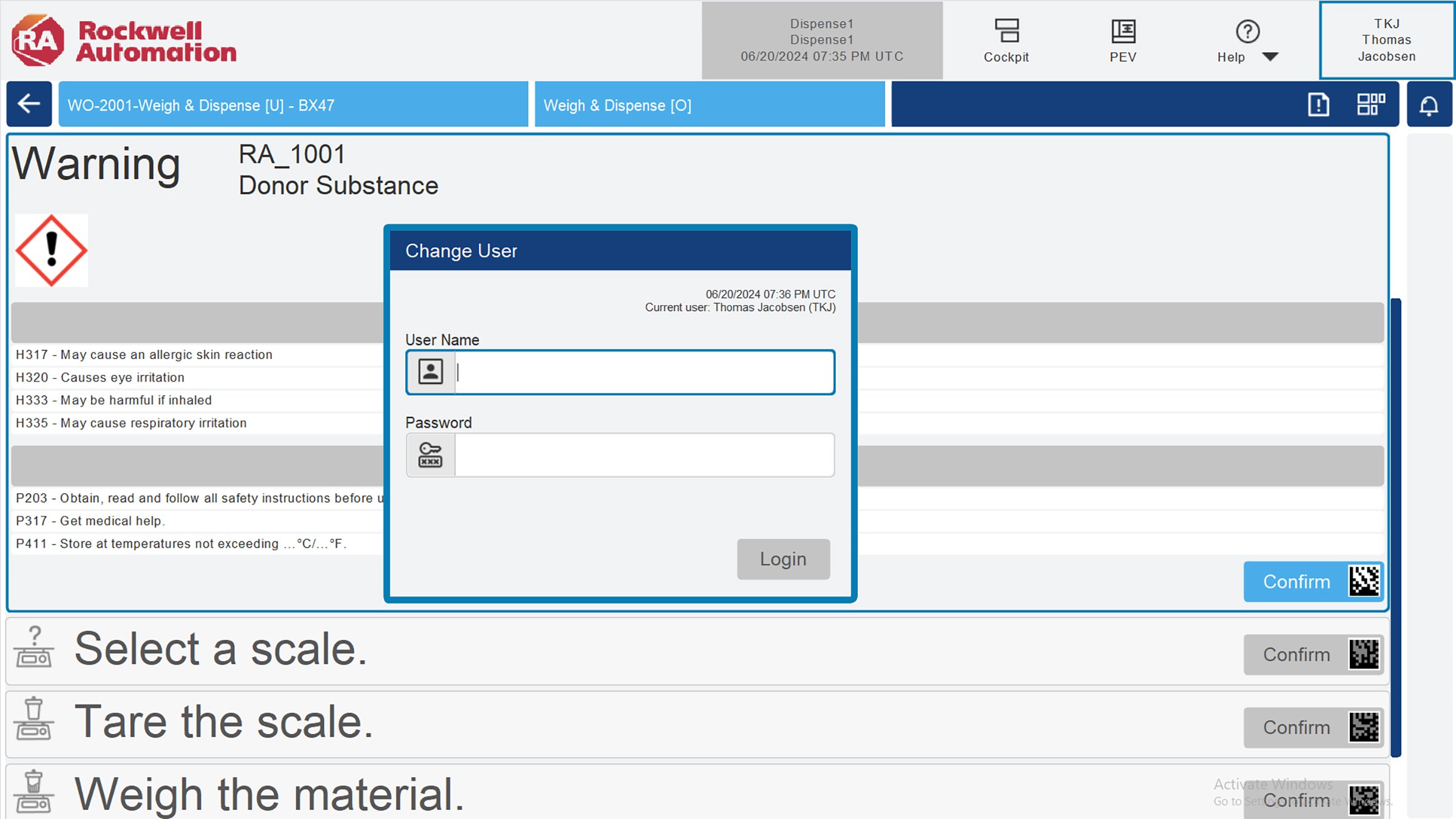
FactoryTalk® PharmaSuite® is the leading MES solution for the life sciences industry. It offers organizations a suite of digital process integrations catered to any stage of maturity. From tightly coupled process orchestration, high-speed adaptability for therapeutics, or a transition from traditional paper-based methods to a modern, digital "paper-on-glass" approach, PharmaSuite is a versatile solution designed to meet diverse organizational requirements. Additionally, it seamlessly provides electronic batch records with precision guidance and intuitive workflow authoring. With Nymi, authentication-based workflows in PharmaSuite are transformed into a passwordless, contactless, and hands-free wrist-tap, further enabling users for greater productivity and ease of use.
In the Life Sciences industry, ensuring compliance is everything. Getting a therapy from inception to a patient’s bedside requires a herculean effort with countless steps, each requiring authentication to achieve drug safety. The rise in biometrics for manufacturing is centered on enabling operations using electronic systems to streamline compliance throughout a process. By utilizing authentication methods like a wearable device on your wrist, organizations can expedite their execution time across processes and foster greater regulatory compliance.
Benefits of Biometric Authentication
Security
Biometric authentication devices work by recognizing a specific ID associated with the device that is linked to a username and password to instantly log a user in or validate a process step. All of this is done through encrypted communications between software, removing the risk of credential misappropriation at an enrollment station or contamination from PPE removal/adjustments in a cleanroom setting.
Efficiency
Working with a biometric device gives you the ability to sidestep cumbersome processes by enabling e-signatures. These devices allow expedited EBR execution reducing the time it takes to perform signatures during order execution and enabling recipe designers to streamline recipe releases with minimal keystrokes, taking what can often be a 10-30 second process down to nearly a second. These small, incremental gains compound over days, weeks, and months, tallying to a significant time savings. This also facilitates faster batch releases with quality personnel now able to sign across the batch release process in an expedited manner.
While the benefits of using this technology in your day-to-day operations are clear, there are also challenges that must be considered before taking the leap.
Organizational Change
Adjusting to a new way of executing operations takes time and effort. Staff will be used to conducting workflows in a certain way. Adopting a new technology will require an adjustment period, and re-learning of specific job functions. Critical activities such as gowning, wash down, signature prompt handling, charging, and storage procedures should all be considered with the addition of biometric authentication methods.
System Integration
Not all systems are created equally. Integrating biometrics as an extended logon method is not always as easy as originally envisioned. Some systems lack openness to biometrics and may require customizations or updates to achieve compatibility. Many biometric vendors provide extensible software with their devices, allowing software vendors to integrate with the ecosystem more easily. Considering system topologies across the site or organization when planning for these integrations will save time and effort in the long run.
Cost
As a waterfall of the challenge above, the software and hardware required to successfully integrate biometric authentication into your day-to-day workflows comes at a cost. Biometric authentication software vendors link to an Active Directory, enabling an organization’s central login repository to pair with, verify, and authenticate usernames and passwords through biometric devices. The additional costs associated with biometrics devices can often be overshadowed by the significant improvement in process execution efficiencies.
Rockwell capabilities
Nymi and Rockwell Automation have partnered to deliver a full-feature integrated solution for FactoryTalk® PharmaSuite® and ThinManager®. Organizations can now use the Nymi BandTM, a next-gen biometric authentication solution you wear, for password login and e-signatures across these Rockwell technologies. With this partnership, end-users in highly regulated plant floor environments are digitally empowered with a wearable solution that makes work simple, secure, and productive across devices, applications, and networks.
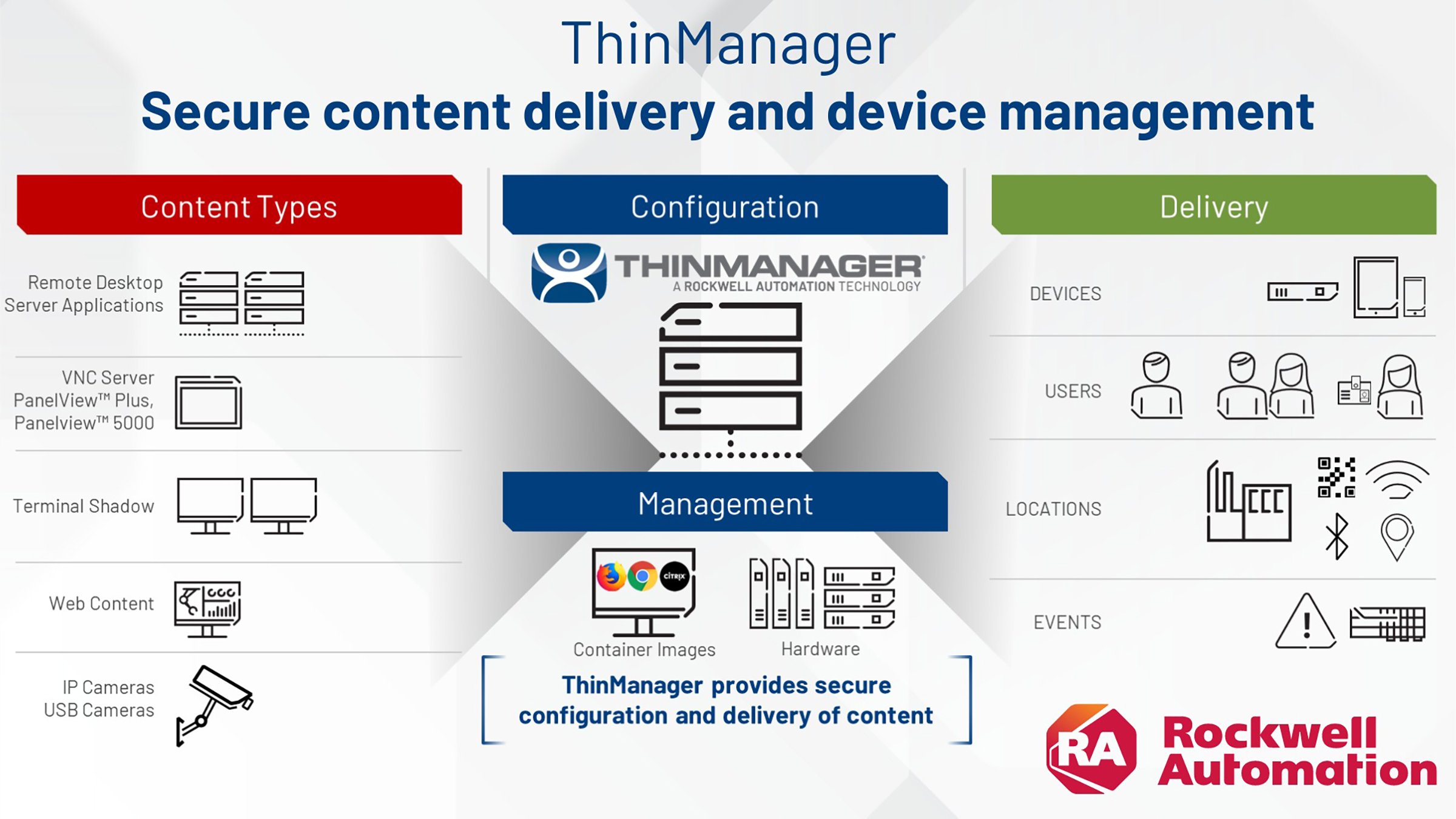
ThinManager®, a Rockwell Automation technology, is an industrial content delivery and client device management platform designed for modern factories. ThinManager reduces the cybersecurity footprint of your production operations while providing secure and centralized authentication. Organizations can utilize Nymi’s secure authentication layer with ThinManager for specifically configured access control during login and for e-signatures. Fingerprint biometric authentication devices are also used on ThinManager terminals for operator authentication, some even reading biometrics through the gloves of the operations team benefiting from the productivity savings.
The future of wearables and biometrics
In our day-to-day lives we already see biometric authentication built into staple technologies, whether that be facial recognition to unlock your iPhone or fingerprint scanning to sign on to your laptop, all for the sake of convenience and security. Considering all the signatures required to achieve regulatory compliance throughout the Life Sciences manufacturing process, having the ability to biometrically sign different steps from different personas can have the same effects on your operations. Over time, it may become more commonplace to see these technologies being adapted not only on the manufacturing side of the facility, but in more administrative areas as well. Additionally, as the industry makes the pivot from large and small molecule drugs to more Advanced Therapeutic Medicinal Products (ATMPs), the scale of manufacturing will reduce to smaller, integrated, benchtop or factory-in-a-box style manufacturing. In this instance, biometrics in personal equipment like a phone or tablet, very well may become the standard with wearables laying claim to hazardous, or environmentally sensitive production areas.
Published July 15, 2024