Recommended For You
Challenge
- Contaminations during packaging of sterile injectables was causing double-digit batch loses per year.
Solutions
- Manufacturing Intelligence - FactoryTalk software applications store, organize and display data from control system to automate FDA reporting, present custom reports and ensure quality-control
- Manufacturing Intelligence - Automated Systems Inc. combines industry expertise with system design, configuration, implementation, validation and project management
Results
- Saved at least $250,000 per year by reducing batch losses to zero
- Supervisory remote batch status monitoring allows a single operator to service several processes.
- Reports stored in PDF format allow batch traceability
- Historical data available for trending and process analysis
- Dashboard reports configured with role-based KPIs provide operators and management clear, actionable, real-time information
Background
Many vital medications are in short supply in the United States, a situation that has sparked patient outcry and even congressional hearings. Topping the list of critically needed drugs are liquids that patients receive either by injection or intravenously. In fact, nearly 75 percent of the medications on the federal U.S. Food and Drug Administration's shortage list are liquids, such as chemotherapy drugs, anesthetics and nutritional solutions.
The FDA and industry experts agree that a big part of the problem is the stringent and often complex process required to protect drug purity during production. In 2010 alone, more than half the batches of injectable drugs produced by pharmaceutical manufacturers were thrown out because of quality issues, such as bacterial contamination and industrial particulate impurities.
The story of one manufacturer illustrates the seriousness of the situation – and how companies can use automated technology to help overcome it.
Challenge
Contamination on the packaging line resulted in double-digit batch losses in one year for this U.S.-based producer of sterile injectables. The problem in the process occurred during the transfer of medication from bulk vessels into individual plastic vials using a blow, fill and seal machine.
To ensure all the equipment was sterile prior to introducing the medication, the operator manually pressurized the line by opening and closing a series of 20 to 30 valves throughout the process. This eliminated outside air – and contaminants. But opening a valve out of sequence or at the wrong time could compromise the batch.
Human error wasn't the only problem. A defective valve seal or instrument coupling could also trigger a loss of pressure and threaten the product's purity. The line also lacked an alarm system to signal potential failures, so plant operators didn't know if they had a bad batch until the end-product was tested.
Solution
Searching for a comprehensive solution, the drug manufacturer tapped Automated Systems Inc. (ASI) to upgrade its control and visualization system. ASI created a system to automatically contain contamination on the line, issue alarms when any process metrics varied outside of preset ranges, and track and record key process data to verify the sterility of each completed batch.
The solution relied on a manufacturing intelligence strategy based on Rockwell Automation hardware and software. Leveraging a new Allen-Bradley® ControlLogix® programmable automation controller, ASI engineers designed a system that predetermines all processing and sequencing steps, including the crucial pressurization procedure.
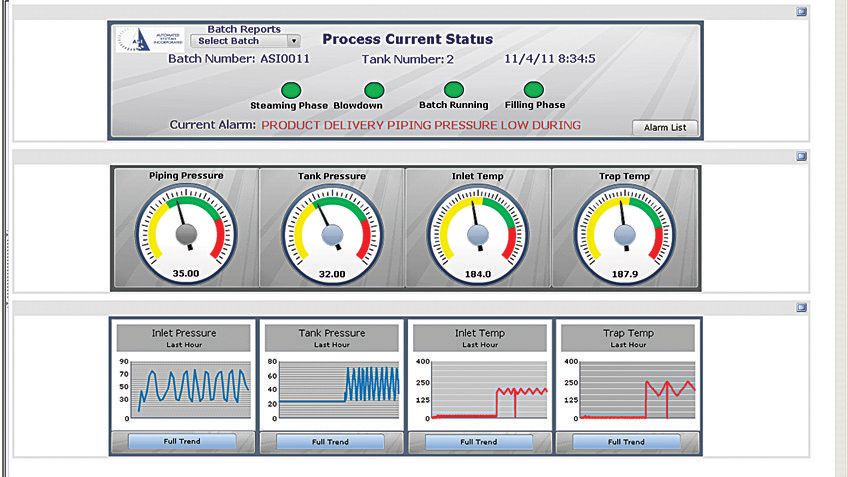
The system incorporated FactoryTalk software from Rockwell Automation, including a human-machine interface (HMI) running FactoryTalk® View Site Edition (SE) software. The HMI shows the real-time state of the system in a sophisticated mimic screen. This screen – which also can be accessed via a secured web-browser for remote monitoring – gives operators a detailed overview of the status of every valve across the system, along with pressure and temperature readings.
This enables the operator to quickly and correctly perform the right operation with optimal awareness of the entire system. FactoryTalk View SE software prompts the operator to perform certain actions and verify variables at each step in the process. In the event of a warning or alarm, the system automatically identifies the potential failure site, isolates the product there and re-sterilizes the line.
For improved record-keeping and simplified reporting, ASI incorporated FactoryTalk Historian and VantagePoint EMI software into the new system. Together, the applications track and report all critical process parameters and alarms. Now, operators have real-time access to critical production data from automatically generated interfaces, dashboards and reports, so they can defuse any potential production issues before they occur. And business managers can evaluate historical data to make better-informed decisions.
At the end of each operation cycle, the new system automatically generates a report listing any alarms or conditions affecting sterility. If operators see an anomaly, they can refer to the historical data to pinpoint exactly where the error occurred and quickly work to remedy the situation. Automatically tracking production data also eliminates the need to test each batch at the end of the process.
The reports are stored in PDF format to provide batch traceability. Plant operators and managers use the historical data for trending and process analysis.
FactoryTalk Historian and VantagePoint EMI software also satisfy regulatory reporting requirements. FactoryTalk Historian acts as a safe records repository that allows the manufacturer to comply with the FDA's electronic records rules. FactoryTalk VantagePoint EMI enables the system to correlate dependable supporting data for the batch record, improving compliance with the agency's current good manufacturing practice regulations.
Result
Since implementing the new system, the manufacturer hasn't lost a single batch to contamination. Each finished batch is worth more than $250,000, so the investment in the new system quickly paid for itself.
The company also spends less time and money complying with FDA reporting regulations because the system automatically tracks batch safety.
Patients also benefit, receiving more of the medications they need.
Published May 28, 2015