Vaccines have, understandably, dominated news headlines in recent times, giving the public some insight to the practicalities of developing, distributing and administering life-saving medicines.
For ProjectBinder, a global company specialising in project management for the automation (OT), IT and network fields with operations in Denmark and Spain, finding a simple and inexpensive solution to frictions in the medicines supply process produced a business opportunity with positive human outcomes. Working with a leading producer of vaccines for critical conditions including polio, ProjectBinder created a mobile traceability device that improves the authenticity and trust of medicines in the field while producing massive cost efficiencies across the supply chain.
Supply Chain Risks
The authenticity and trustworthiness of products is largely taken for granted in the western world. Few shoppers of consumer goods or pharmaceuticals in developed markets would suspect tampering or counterfeiting in their purchases.
In the developing world, however, such issues are a real and common risk. News stories such as the Chinese melamine-laced baby milk scandal of 20081 highlighted the devastating effect that tainted goods can have when they infiltrate the supply chain.
When it comes to vital medicines, the risks are even more severe. Counterfeit vaccines account for hundreds of thousands of deaths annually across the globe and cost local governments billions of dollars2. In developing markets where the need is critical and the resources for rigorous inspection are limited, the necessity for verifiably safe medicines is massive.
Searching for a Solution
One of ProjectBinder’s customers, a leading producer of vaccines, has established a formidable reputation in the Life Sciences industry. A pioneer in medicine development, the company employs a complex global supply operations so that its vaccines can be issued to areas of critical need – in a safe and frictionless way. Producing and distributing polio vaccines to countries in the developing world could encounter a variety of logistical and administrative hurdles, opening up risks such as loss, theft or replacement with potentially hazardous alternatives.
The vaccine company wanted to improve the way it managed the distribution process, enabling its vaccines to be tracked and verified at every step from its production facilities through to the hospitals, pharmacists and vaccination clinics where it would be administered to patients. This approach is also desirable for the producer’s customers, such as hospital administrators, who often need evidence of receipt of goods in order to claim financial support from government bodies.
In 2019 the vaccine company began investigating potential solutions available in the market and found several significant problems. Firstly, most solutions it examined needed to be integrated with existing serialisation systems, requiring extensive technical work to implement the changes in its own infrastructure, as well as that of its clients. The second issue related to cost, as integrating a traceability solution end to end would involve replacing and upgrading existing systems, with costs typically in the millions of Euros. There were also administrative costs to consider, which would make sending smaller batches of medicines less feasible, potentially pricing some hospitals in poorer countries out of taking delivery of treatments.
Furthermore, the common solutions on the market posed problems from a mobility perspective. The hospitals administering the medicines would need to setup the tracing systems in a stationary location within their facility, which could prove difficult or bureaucratic in practice. As a consequence, the system could not then be taken out to the field, such as for the purpose of administering to remote communities.
The combination of these factors meant existing solutions were not practical for the company, nor for its customers.
Simple, Yet Effective
Fortunately, the company found a more suitable solution by engaging ProjectBinder. ProjectBinder has deep vertical experience in this area and was able to apply that knowledge in order to come up a unique and creative solution.
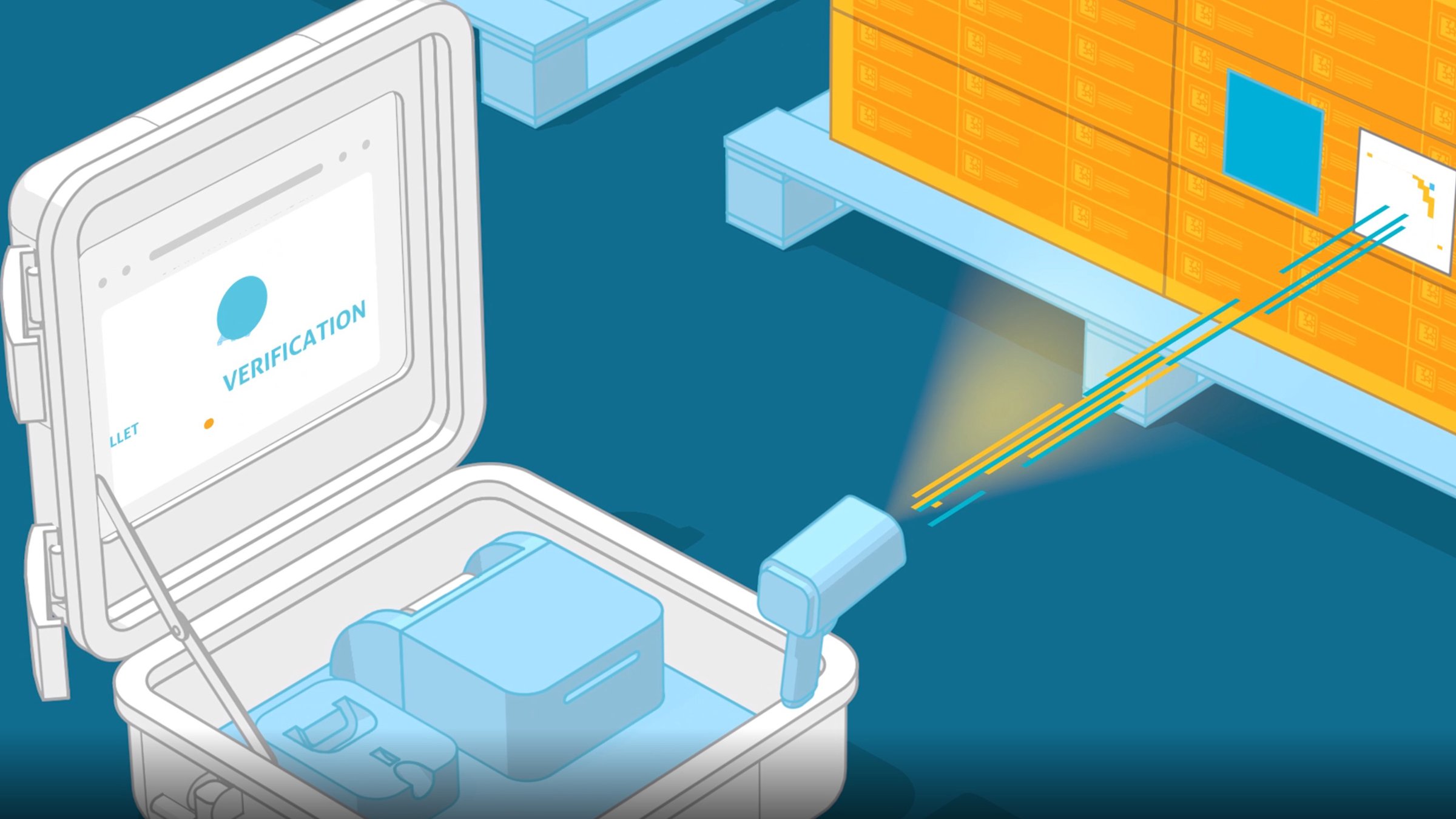
ProjectBinder designed a portable traceability solution named Black Diamond Mobile Commissioning & Aggregation Tool. Taking the form of a 20 kg suitcase-shaped box, it can be easily transported to wherever it’s required at any stage of the supply chain and simply set up without the need to connect to existing serialisation infrastructure. Featuring a scanning device, monitor, control system and label-printer, the solution allows the user to verify the medicine has come from an official source, and acknowledge receipt through a globally accessible, cloud-based data management system called TraceLink.
According to Martin Petersen, General Manager at ProjectBinder, Black Diamond filled an important gap in the serialisation market. “We were challenged to create an agile, flexible and robust method for our customer to commission and decommission product units, ranging from small bundles through to larger cases or pallets. This normally requires a substantial investment in OT infrastructure that would only be economical for large unit quantities, so we had to really think outside the box to overcome some of the evident design, cost and practical usage constraints.”
The solution uses leading-edge technologies from Rockwell Automation, for whom ProjectBinder is recognised as a system integrator partner. By using an Allen-Bradley® CompactLogix™ 5480 programmable automation controller (PAC), the serialisation component can be integrated together with the PAC logic, offering greater control over the process and increased traceability.
“We were looking for a control system that could collect data at speed and the 5480 model really delivered. With high-performance control and data throughput, the real-time controller integrates easily with the other components and provides strong reliability,” Martin added.
Any participant in the supply chain process, including in the procurement, packaging line, shipping and decommissioning phases, can scan the barcode to see what steps have been taken along the chain and quickly identify if there’s a risk of tampering in the medicine’s delivery. This is important from the perspectives of health and quality and also from a compliance standpoint, ensuring that the distribution aligns with regional and local regulations.
Moreover, the solution has been delivered at a significantly lower cost than alternative solutions. The end user was able to retain its existing IT infrastructure and operate the solution at a separate layer to integrate with its Level 4 and Level 5 systems, allowing implementation to be performed at a fraction of the cost.
Rolling Out, Rolling On
In the time since ProjectBinder implemented the solution, the end-user company has now seen significant progress. The solution has been rolled out across multiple sites and used extensively, particularly in hard-to-reach areas where mobility is a valuable feature.
Martin is proud of what’s been achieved to date, and hopes that it’s only a taste of the variety of potential applications for Black Diamond, especially as today’s consumers become more conscious of the need for verifiable product origin and safety.
“To have a solution we developed be used for the purpose of saving lives and helping healthcare workers, who are providing vital treatments to gain peace of mind in carrying out their work, is immensely satisfying. We are talking to our customer about how Black Diamond can be further scaled and hope to see new use cases for the solution emerge in other sectors too.”
Additional Information
The results mentioned above are specific to ProjectBinder’s use of Rockwell Automation products and services in conjunction with other products. Specific results may vary for other customers.