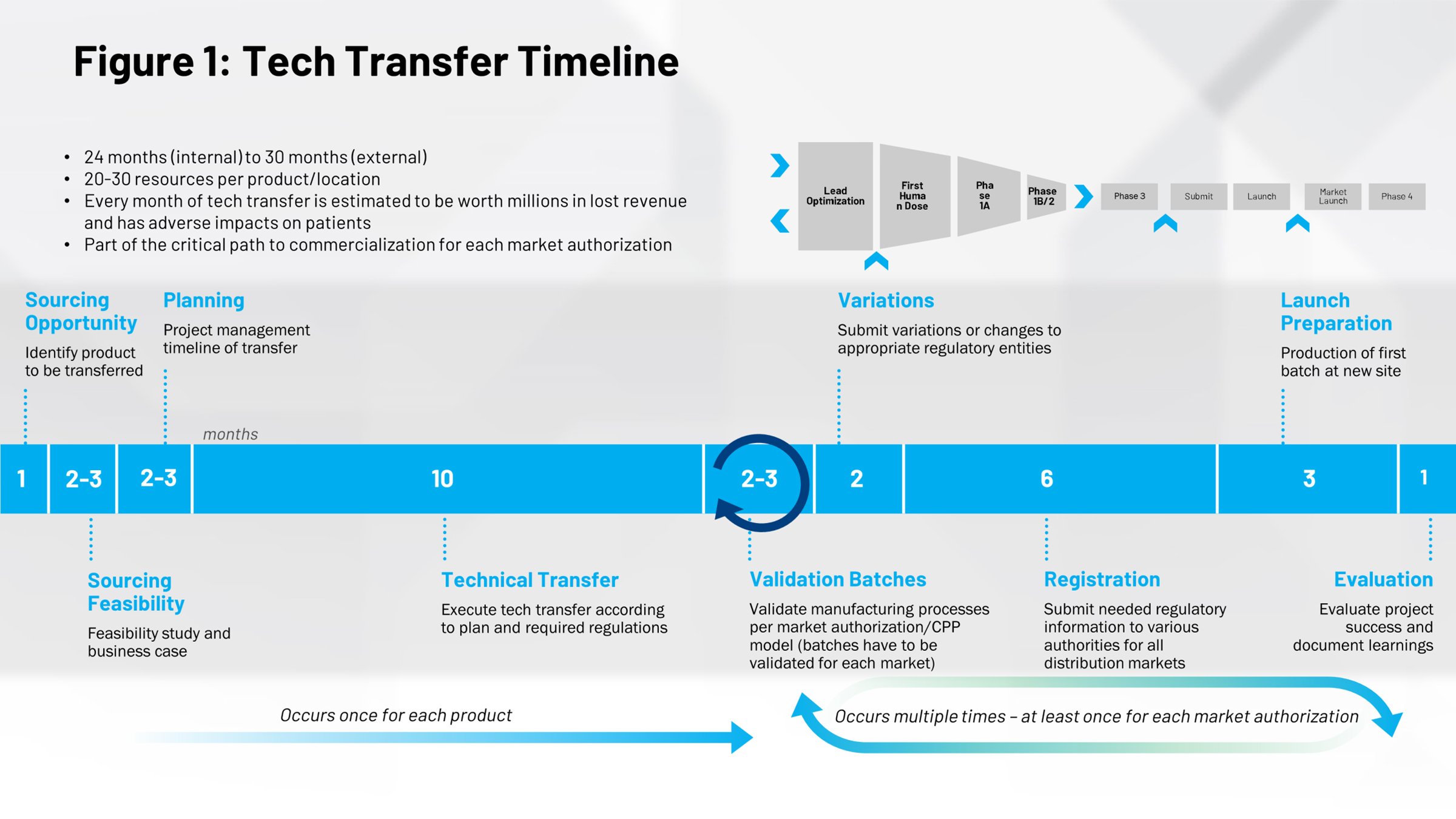
Looking at the timeline of a typical tech transfer event (Figure 1) gives us a different perspective on their impact. The first half covers the process of bringing drugs to market, 10 months of which is the average amount of time required to scale up a recipe and reach the commercial batch stage. There’s a tremendous opportunity here. If you can execute this process more efficiently, you save time that will get drugs to market faster and earlier in the patent life so you have more time under patent protection.
Everything on the right half of the timeline is repeated cycles that happen when you perform additional tech transfers, such as internal transfers from other parts of your organization to a separate site around the world. If you can digitize these processes, if you can make them more efficient, more repeatable, there are significant potential savings in time and effort.
What’s stopping us?
So why haven’t we done this right? What are the problems that have held us back? What are the challenges that we need to overcome? One is the data getting processed and created.
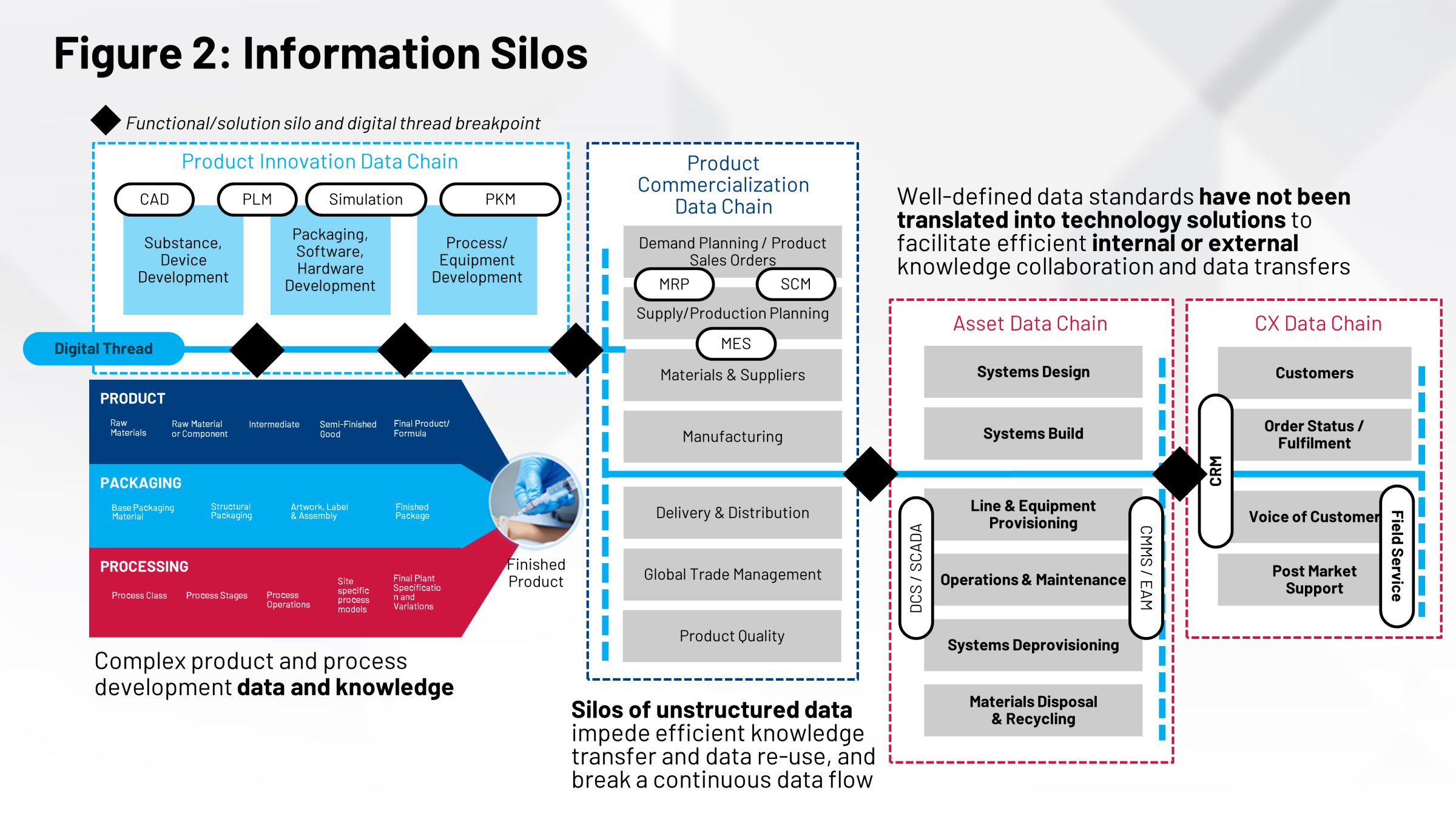
If you look at chemistry and manufacturing control (CMC) processes, they take the critical quality attributes (CQAs) we know of, and then we try to develop the processes and the key process parameters we need to be able to hit those CQAs. But the CQAs in process development and the process recipe cycle ultimately need to get built into your regulatory filings. They need to get built into your validation batches and your registration batches.
The groups within your organization working on those areas will need that information to prove that your recipes really work. Then there are post-approval concerns around change management, site-to-site management and recipe development that need to align.
Often, these groups aren’t working together off the same piece of information. There is no common repository, no single system of record for all the information that goes into manufacturing those products. Ultimately, because you don’t have that single version of the truth, it impacts your ability to perform effective tech transfer, process validation, site-to-site comparisons and many other critical actions in the most efficient way.
Not having those things working together and in coordination impacts the execution systems that help make your products. Your manufacturing execution system (MES) and your distributed control system (DCS) need that information. In today’s world, we do that through manual processes: paper records, manual transfers and the sheer horsepower of human resources getting those things done.
On the same page
Another way to look at this is you have definitions around your packaging, your process and your product that are necessary to perform critical functions. The labeling on your product, the content on the label, the product design, the packaging design — they’re all key pieces of information, and they’re all developed by different groups.