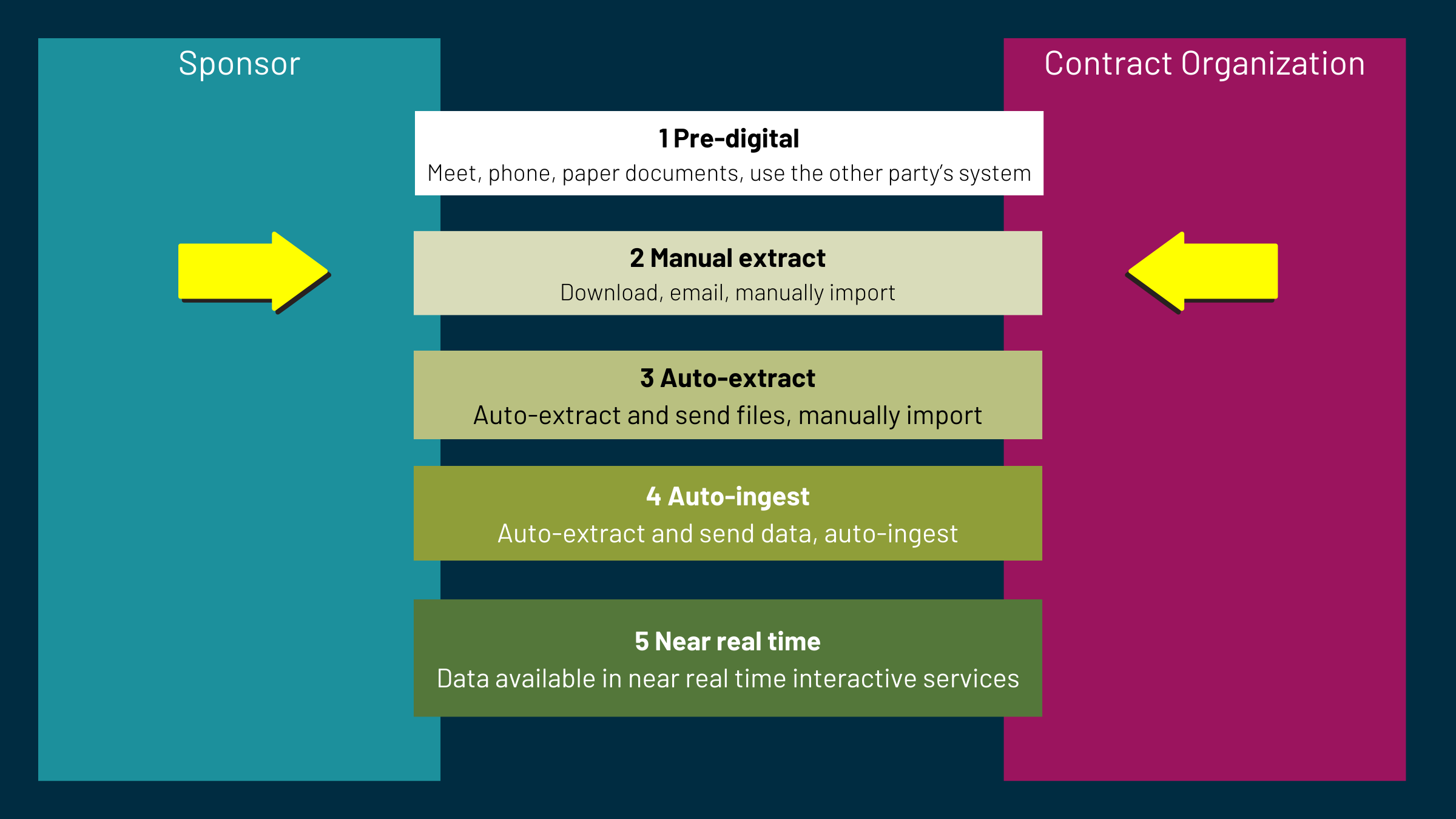
What do you see in a level 2 tech transfer?
Manual extract is where humans take information from one system and send it to other humans who import or enter it into their systems. Most simply, files are emailed, which is not guaranteed delivery, it is difficult to audit, and susceptible to security breaches. At its most advanced, there is a shared folder with no structure and requires manual manipulation.
Level 2 tech transfers are time consuming. A Significant amount of effort goes into developing bioprocesses, especially for complex biologics and cell therapies. Extensive process documentation is needed which may include:
- Process descriptions, setpoints, and operating ranges
- Equipment specifications
- Material descriptions, specifications and suppliers
- In process controls, and sampling plans
- Analytical methods and Quality attributes
- Buffer recipes, etc.
Subject matter experts are needed to manually review all documentation and then extract and transcribe important process details in the format being used at the manufacturing site. Errors in transcription can lead to failed batches or data integrity issues. Translating parameters to new equipment at a larger scale or with different control capabilities also poses a challenge. Finding a way to identify these gaps early and decide on the path forward is important for future success.
If you can move away from level 2 manual extraction and “level up” toward auto-extracted and auto-ingested data, you can significantly improve the tech transfer process.
To achieve this, you must build a foundation through digital transformation.
What does this look like?
Faster. Digital transformation will increase speed with auto data extraction from unstructured documents.
More Robust. Reduce errors with a structured system of record for process definition composed of common recipe building blocks that can be propagated to manufacturing systems in a controlled manner.
Efficient. Facilitate communication with a single platform for sharing knowledge and data, creating well-defined recipes, and achieving appropriate oversight through the entire process.
How do you get started?
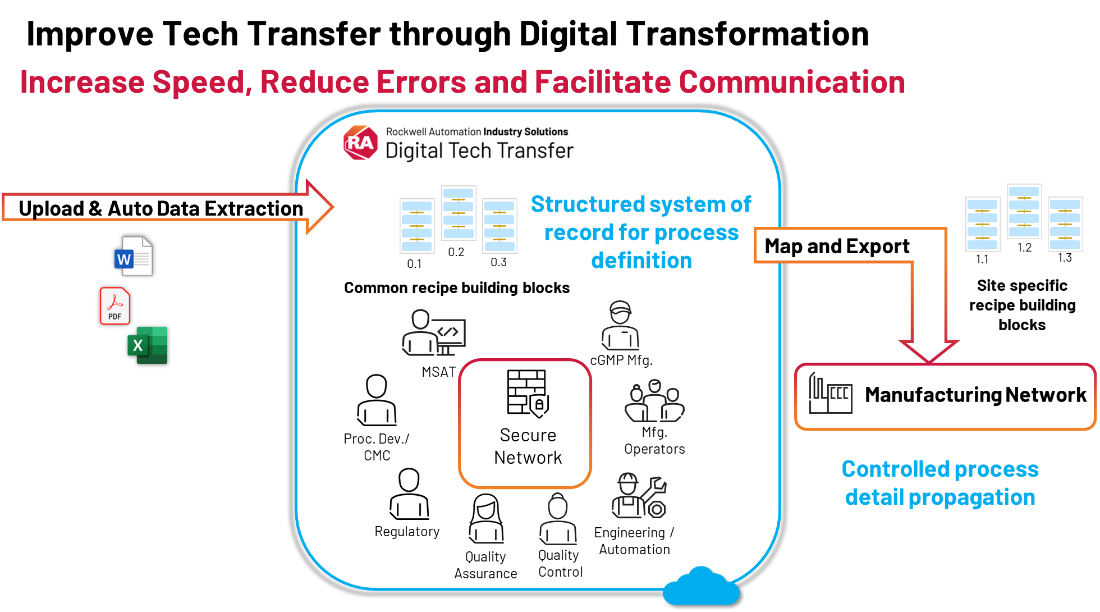
You must establish a secure, collaborative network.
- This platform would create space so the tech transfer team has a place for document submission and controlled revisioning
- The location can act as a repository of knowledge that is a single source of truth for the process details.
You must get rid of paper.
- At this point, you can upload source process documents to the secured network for collaboration and tracking. This will serve as your tech transfer package.
- Then, you can take those documents and automatically extract relevant process details with cutting edge technologies like generative AI. This allows converting from unstructured process documents to structured digital datasets.
- The datasets make up the core of your process recipe with critical information such as the process parameters, ranges, material and equipment specs, etc.
Digitize manufacturing plants.
- A digitized model of your plant that can describe available equipment and capabilities.
- Increasing transparency allows the tech transfer team to more easily choose the right site.
- Begin to digitally map out gaps by comparing the existing process information to digital plant assets and their processing capabilities.
Create tailored recipes.
- Create common recipe blocks from converted digital datasets in a standardized format such as S88
- Configure the general recipe workflow by linking together the common recipe blocks.
- Map the common recipe building blocks to site-specific equipment to consider equipment attributes and scale dependent parameters.
Link to manufacturing network.
- Propagate site-specific recipes to other systems in the manufacturing network for further editing and execution.
- This includes systems such as Manufacturing Execution System (MES), Distribute Control System (DCS) and Batch Execution System (BES)
- This propagation can drastically reduce the errors and time it takes to deploy an automation system that provides further benefits.