Note: This blog references the January 2026 compliance deadline as it was created prior to the announcement from the FDA with its intention to extend the compliance deadline to July 2028.
Are you Ready to Comply with the FDA’s FSMA Requirements for Section 204?
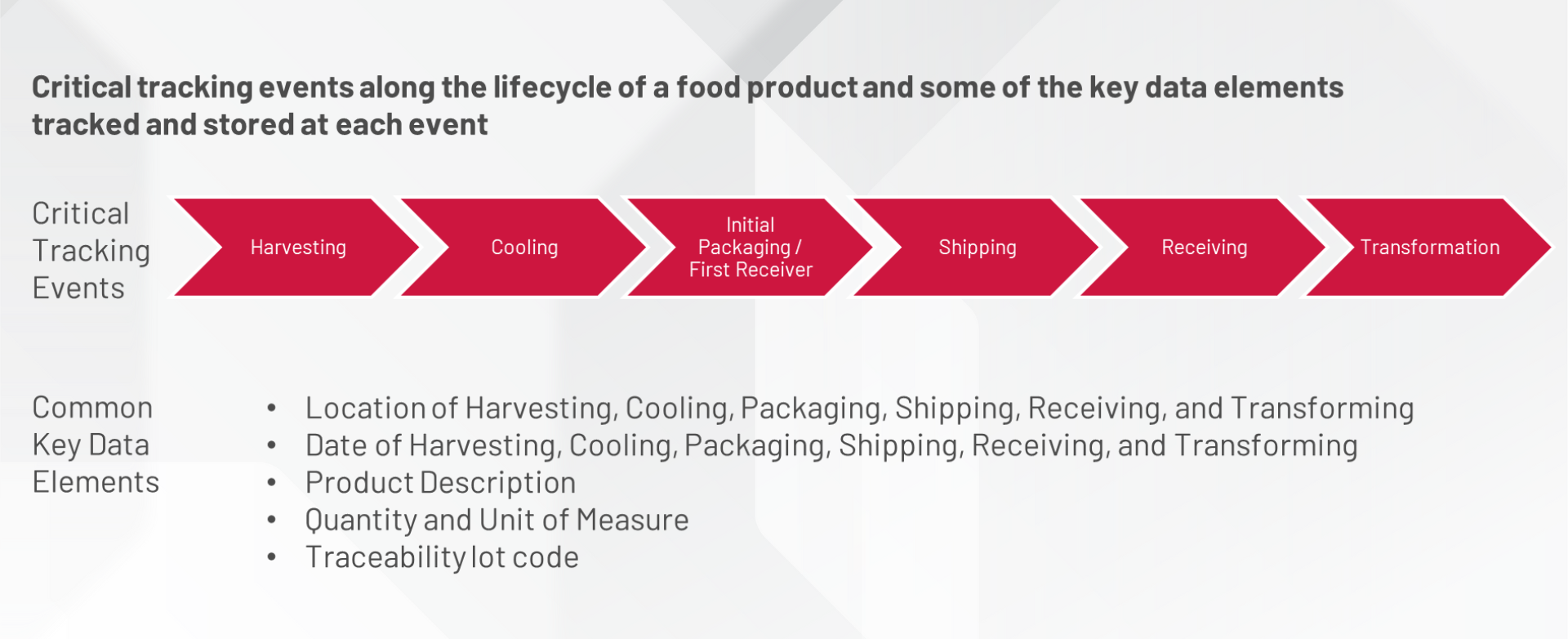
As a food and beverage manufacturer, you know the importance of food safety, but this new rule takes it a step further, requiring the ability to maintain records containing key data elements for critical tracking events along the lifecycle of a food product.
As part of Section 204 of the FDA Food Safety Modernization Act (FSMA), the final rule on Requirements for Additional Traceability Records for Certain Foods (Food Traceability Final Rule) has been published and requires compliance by January 20, 2026.
This affects domestic and foreign firms producing food for US consumption, and it requires a Traceability Plan for all entities throughout the food supply chain. While many larger companies may be compliant in keeping with industry best practices, smaller or mid-size companies may not have everything in place yet to track food products on the Food Traceability List (FTL), from farm to table.